Charge to Review and Adjusting Medications in a Clinic
- Study protocol
- Open Access
- Published:
Supporting clinical rules engine in the aligning of medication (SCREAM): protocol of a multicentre, prospective, randomised study
BMC Geriatrics book 17, Article number:35 (2017) Cite this article
Abstract
Groundwork
In the nursing home population, it is estimated that i in every 3 patients is polymedicated and given their considerable frailty, these patients are especially prone to agin drug reactions. Clinical chemist-led medication reviews are considered successful interventions to ameliorate medication prophylactic in the inpatient setting. Due to the express available evidence concerning the benefits of medication reviews performed in the nursing abode setting, nosotros propose a report aiming to demonstrate a positive upshot that a clinical decision back up organisation, as a health care intervention, may have on the target population. The primary objective of this report is to reduce the number of patients with at least 1 event when using the clinical decision back up system compared to the regular care. These events consist of hospital referrals, delirium, falls, and/or deaths.
Method/Pattern
This study is a multicentre, prospective, randomised study with a cluster group design. The randomisation will exist per main nursing domicile physician and stratified per ward (somatic and psychogeriatric). In the intervention group the clinical determination support system will be used to screen medication list, laboratory values and medical history in order to obtain potential clinical relevant remarks. The remarks will be sent to the master md and feedback volition exist provided whether the communication was followed or non. In the control grouping regular care volition be applied.
Word
Nosotros strongly believe that by using a clinical decision support arrangement, medication reviews are performed in a standardised way which leads to comparable results between patients. In addition, using a clinical decision back up system eliminates the fourth dimension factor to perform medication reviews equally the major bug related to medication, laboratory values, indications and/or established patient characteristics will exist directly available. In this way, and in order to make the medication review procedure complete, consultation within healthcare professionals and/or the patient itself will be time effective and the medication surveillance could exist performed around the clock.
Trial registration
The Netherlands National Trial Register NTR5165. Registered 2nd Apr 2015.
Groundwork
Polypharmacy is defined as the use of more than a certain number of drugs irrespective of their ceremoniousness [1, 2]. In the Netherlands, it has been defined as the chronic apply of 5 or more drugs from dissimilar therapeutic groups or subgroups [3]. In the nursing home population, it is estimated that 1 in every 3 patients is polymedicated [four] and given their considerable frailty, these patients are extra prone to adverse drug reactions. In addition, their direction is often challenging given the comorbidities and/or circuitous organ role impairment [one,six,7,, 2, 5–viii]. Furthermore, polymedicated patients are likewise at risk of suffering from inappropriate prescribing in the form of underprescription. Information technology has been demonstrated that underprescription increases significantly with the number of medicines used [9]. This situation strengthens the need for routine medication reviews and treatment optimisation [x, eleven].
Clinical pharmacist-led medication reviews are considered successful interventions to improve medication safety in the inpatient setting. However, there is limited available evidence of the effects concerning comparable interventions performed in the outpatient setting [1, 12, 13]. In add-on, few studies have evaluated health related outcomes resulting from clinical chemist interventions in nursing homes. Nevertheless, it has been suggested that most of these studies had major limitations: no command grouping, no clinical issue measures, inadequate employ of nursing staff to influence change, and data analysis by drug use per provider rather than drug use per patient [6, 8, x, 11, 13, xiv]. Some of these studies were randomised controlled trials performed in the nursing home setting past ways of a clinical pharmacists-led medication review; some of them measured the effect of multidisciplinary case conference [half dozen, xv]. In other studies, pharmacists performed the medication reviews and sent suggestions to physicians [8, 10, 16]. Even so, some improvements in patient outcomes have been described [8, 10]. The results from these studies are difficult to compare due to the big differences with respect to the interventions applied, the outcomes studied, the settings, and elapsing of follow-up after the medication review.
Pharmacotherapy optimisation in nursing abode patients relies on the development and cess of novel healthcare interventions [xi]. Information technology is suggested that performing a standardised intervention could potentially pb to a successful medication review; this intervention necessitates pharmacists and physicians collaboration, information technology should include the complete medical and drug history, and fully availability of laboratory values should be guaranteed [10,18,, 12, 13, 17–xx].
In the netherlands, the Dutch Healthcare Inspectorate (IGZ: Inspectie voor de Gezondheidszorg) expects that a medication review is performed by a doctor and a pharmacist in all residents of nursing homes yearly; even so, this communication implies substantial actress workload for the involved health care professionals. In addition, in this medication review the information given by the nursing staff and the patient him/herself should also be taken into account.
From our experience, medication reviews involve a time consuming procedure that takes an average of 90 min per patient. When because a nursing dwelling of near 150 patients, 450 h a year would take to be dedicated at performing medications reviews.
In daily practice, this unfortunate situation leads to a non-continuous medication review process implying major consequences that may range from an increased number of potential agin drug reactions, unnecessary hospitalisations and, at worst, expiry.
Computerised clinical decision back up systems (CCDSS) can exist defined equally conclusion-aiding tools which provide wellness care professionals with clinical cognition and patient-related information, intelligently filtered or presented at appropriate times, then every bit to heighten patient intendance [twenty,21,23]. Within the SCREEN project (Supporting Clinical Rules in the Evaluation of Elderly patients with Neuropsychiatric disorders), a CCDSS named Clinical Rule Reporter (CRR) has been adult. This system currently analyzes, independently of the applied prescribing software, the medication used by patients in relation to their co-medication, the laboratory data (including renal function), and other relevant clinical data like diagnosis and comorbidities [24]. The CRR combines the clinical rules (algorithms) with the medication list, patient characteristics and laboratory values of the patients in order to obtain concrete advices. These clinical rules or algorithms work with triggers that place drug related bug like renal or liver dysfunction likewise every bit the demand of new medication (tummy protection or laxative agents), the necessity to stop a certain drug or decrease the dose co-ordinate to age, etc.
Due to the lack of testify concerning the benefits of medication reviews performed in the nursing home setting, we suggest a study aiming to demonstrate a positive effect that the CRR, every bit a wellness intendance intervention, may accept on the target population. This population consists of older people (≥65 years) with a high take a chance of suffering harm when using inappropriate drugs. By this we mean people living in nursing home facilities; these people ofttimes suffer from polymedication amongst other risk factors such as multimorbidity, impaired cognition, renal dysfunction, and increased risk of falling.
The primary objective of this study is to reduce the number of patients with at least ane event when using the CRR compared to the regular care. These events consist of hospital referrals, delirium, falls, and/or deaths. Secondary objectives will also be evaluated, including: the analysis within a middle to account for possible differences concerning regular care, the separate analysis for psychogeriatric and somatic wards, the analysis for medication related events (infirmary referrals, delirium, falls, and/or deaths), the split up analysis for each of the parameters included in the combined endpoint, the analysis of the quality of life EQ-5D, the analysis of the MAI (Medication Appropriate Index), and finally, a cost analysis.
Methods/Design
Written report design
The Supporting Clinical Rules Engine in the Aligning of Medication (SCREAM) report is a multicentre, prospective, randomised study with a cluster grouping blueprint. The randomisation volition be per main nursing home medico and stratified per ward (somatic and psychogeriatric). This study volition exist blinded for physicians and for patients; physicians will be emphatically requested not to discuss with each other about the report to avert bias. The study follows the CONSORT guidelines.
Overall study design
In order to apply the CRR, the nursing homes will accept to provide the medication list, the patient characteristics and the laboratory values for each patient in a digital format.
Taking into account the extra workload for the investigators, in that location volition be a predefined day for each nursing home to ship the files: nursing home A sends the files on Mondays, nursing home B sends the files on Tuesday, and so on.
All nursing homes volition ship the patient information both for control and intervention groups.
The randomisation will be performed past two of the authors (BvO, CMG). The randomisation volition exist per main nursing home physician and stratified per ward. Physician A will be randomised in the control group and physician B on the intervention group, taking into account that the amount of patients in each group should exist approximately the aforementioned.
Intervention grouping
The datasets volition exist screened through the CRR on a weekly basis. The letters delivered by the CRR will exist sent via post to the specific physicians. Each remark volition exist sent on a separate mail service in a standardised mode. In response to the written report, the md will send a feedback bulletin within 36 h indicating, in a standardised fashion, whether:
-
the advice was non followed
-
the advice was followed
-
the advice was inverse.
After receiving this feedback, the investigators will process it in the CRR, in order to create the database for the study.
Additionally, regular care will be likewise applied. That is according to the Dutch Healthcare Inspectorate, a yearly medication review with a physician and a pharmacist, fifty-fifty though at that place is a substantial variation [25], For the centres included in this study there are no dedicated clinical pharmacist working in the nursing dwelling.
Control group
In the control group patients volition receive regular care (yearly medication review). In addition, these patients will also be screened using the CRR to obtain data that could serve for hereafter evaluations within the project (for instance to compare how many advices would have been sent from the control grouping, the difference in remarks, etc.). However, this screening will be performed via a filter and the investigators will neither meet nor evaluate whatever remark. These alerts volition simply exist unblinded at the end of the study.
In improver, for both control and intervention group, the physicians volition report whatever events including: infirmary access, specialist visit, emergency department visit, falls, delirium and decease, via a questionnaire. This questionnaire will also include questions to know whether a medication review has been performed and how much fourth dimension this medication review toll. Physicians will as well report if there is any new patient. These electronic questionnaire will be sent by Google Drive via email weekly. At the terminate of the study, the physicians in the intervention group volition receive a mail asking how much time, in average, they demand to answer the remarks which are sent from the CRR. Figures 1 and two.
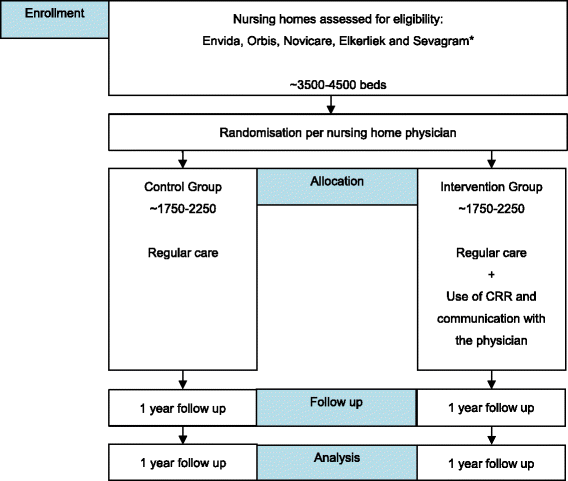
Schematic study pattern. *Other possible centres Amsterdam and Nijmegen
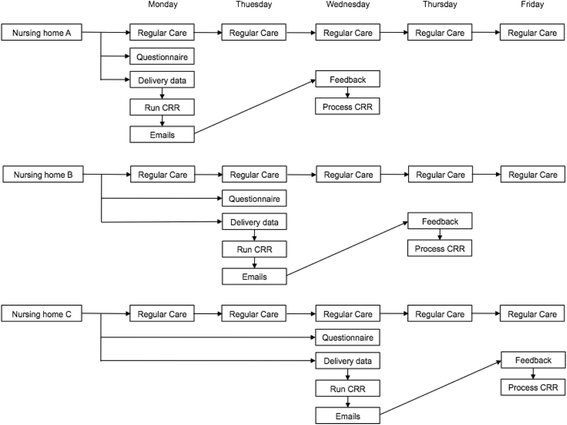
Study schedule
Endpoints
Main endpoint
The main outcome variable in this written report is the proportion of patients with at least one of the events, including hospital referrals (i.east. referral to a specialist, emergency section visit and hospital admission), delirium, falls, and/or deaths. All these events will be reported by the nursing home physician via the electronic questionnaire. To this terminate the written report will assess the differences between regular care (control group) and regular care + CRR (intervention group).
Secondary endpoints
Every bit secondary endpoints, the same result variable volition be used to analyse the possible differences between institutions, to separately analyse psychogeriatric and somatic wards, to analyse the medication related events, and to separately analyse each of the parameters included in the combined endpoint (hospital referrals, delirium, falls, and/or deaths).
In gild to get this information, physicians will be asked to report whatsoever events including: hospital admission, polyclinic visit, emergency department visit, falls, delirium and decease. These questions will be asked via an electronic questionnaire via Google Drive. The assessment of whether the event is or could be drug related or non will be done exclusively past the physician.
The quality of life will be measured using the EQ-5D questionnaire both for patients in the control grouping and patients in the intervention group. The questionnaire will exist performed at the cease of the study (i.e. after ane year follow-up), both for psychogeriatric and somatic patients. The results will be compared betwixt intervention and control group. For both patients groups a caregiver/nurse will answer the questionnaire.
In improver the analysis of the MAI and the cost evaluation will as well be performed for both control and intervention grouping.
Setting
Nursing homes in the Netherlands will exist invited to participate in the written report; these nursing homes should exist able to evangelize the medication data and the laboratory data electronically. In instance the information would come up from the infirmary in the neighbourhood, this hospital would take to agree on providing the data. If a nursing home meets these requirements, information technology is eligible for participation in the present study.
Population
Nursing home residents; the total study population is estimated to take a total of 3500–4500 patients. This broad range in corporeality of patients comes from the fact that patients will not be included singly simply equally complete nursing homes. In addition, plenty patients should be included to ensure reliable results taking into account possible loss to follow up.
Inclusion criteria
Residents living in a nursing home in kingdom of the netherlands.
The nursing homes are able to deliver the medication and lab information electronically.
Participating centres
Zuyderland Medical Heart in Sittard-Geleen (coordinating middle), Envida in Maastricht, Sevagram in Heerlen, Elkerliek in Helmond, and Novicare in different locations. Other centres volition be invited and included when the requirements are fulfilled (Amsterdam and Nijmegen).
Randomisation, blinding and handling allocation
Randomisation
per main nursing home physician. The randomisation will be stratified per ward (somatic and psychogeriatric). In example the physician would be absent-minded the suitable option will be followed:
Absence ≤ 6 weeks intervention group: the mails with the messages obtained from the CRR will notwithstanding exist sent to the main doctor. If the replacing medico would besides participate in the study, he will not become any mails for the grouping of patients for which he/she is the replacing physician during this menses. It is assumed that if the replacing dr. is included in the intervention group, he could apply the mails from his ain grouping to all patients. If the replacing physician is included in the control group, it is assumed that no interventions will be performed.
Absence > 6 weeks intervention group: the mails will be sent to the replacing physician; if the replacing physician would exist i of the physicians already included and randomised in the control grouping, the replacing physician will get the mails only for the patients in the intervention grouping.
Blinding
Blinded for patients; In addition, physicians will exist emphatically requested not to talk nigh the emails.
Treatment allocation
If a patient dies or moves to another establishment, the replacing patient will not take over the place in the study. Death is one of the endpoints for the study and so the study would be completed for that patient; moving to another establishment will be considered every bit loss to follow-up. To account for these patients the physicians volition have to report every fourth dimension a new patient gets in the nursing home, in this way a filter can be applied to not analyse these new patients.
Time schedule
Recruitment started in June 2013; the target population, 3500–4500 patients, is expected to be accomplished in June 2016. The different centres can start with the study at different times. Each centre volition be followed for a menstruation of 1 year and afterwards the data analysis will get-go.
System
Each participating heart has provided a contact person who volition be in charge of coordinating the report in their centres. The investigators have regular contact with these coordinating people to confirm the fulfilment of the inclusion criteria, the adherence to the study protocol, and to provide support or boosted information when necessary.
Cost analysis
A cost analysis will be performed for both groups (control and intervention).
Hospital costs
The assay will take into business relationship the number of hospitalisations or hospital referrals, consisting of personnel (physician, nurse, pharmacists, etc.), material and equipment costs. These costs will be based on study patients records and standard rates.
Costs outside the hospital
This analysis will also take into account the healthcare costs exterior the infirmary similar the addition of new medication.
Sample size calculation
Adding of the full number (one event per patient). The aim is to reduce the number of patients with at to the lowest degree ane event with 25% by using the CRR compared to the regular intendance. These events consist of medication related hospital referrals, delirium, falls, and/or deaths.
In society to calculate the sample size a airplane pilot report was performed. Nursing homes physicians from the region (Envida and Zuyderland) have informed, via an electronic questionnaire, about any hospital referrals, delirium and/or falls within their patients. In addition, they stated whether these events could exist medication related. This airplane pilot report has lasted for 5 months. No patient data was given.
The pilot written report showed a proportion of patients with at least ane outcome (combination of fall, delirium, hospital referral, and death) in the control group of 0.xvi and a mean number of patients per physician of 56.
Assuming a proportion of patients with at to the lowest degree i event during 1 year follow-upward of 0.20 in the control grouping, a 25% reduction by using the CRR compared to regular intendance, i.e. proportion reduces from 0.20 to 0.fifteen, and a 2-sided significance level (α) of 0.05, the number of patients per group required to detect an issue with 80% power equals 906. Accounting for the design effect (randomisation per physician; DE = 1 + (k-ane)*ICC), where we assume an intra-class correlation coefficient (ICC) of 0.01, a mean number of patients per dr. (m) of 56 (pilot study), and a x% dropout rate, the required number of patients increases to 1562 per group.
We causeless a higher proportion of patients with at least 1 consequence in the control grouping (0.xx) than the one found in the airplane pilot written report (0.xvi), because the number of falls were underreported in the pilot study and the follow-up elapsing is now longer, i.e. 1 twelvemonth instead of v months.
Statistical analysis
To account for the cluster randomisation (physicians are randomised, where patients are clustered within physicians), all linear and logistic mixed effects analyses are performed with physicians as random factor.
Principal report parameters
To find a difference in proportions of the chief event (composite endpoint consisting of hospital referrals, delirium, falls, and/or deaths) between the groups (control versus intervention), logistic mixed effects analysis are applied with the following fixed factors: grouping (command or intervention), nursing home organization (Envida, Zuyderland, Sevagram or Novicare), type of ward (psychogeriatric or somatic) and other variables related to the outcome, similar historic period and sexual activity.
Secondary report parameters
For the subgroup analyses (inside nursing dwelling organization or within type of ward), the same analysis method is applied as for the primary result variable, excluding the variable that indicates the subgroups.
For the other endpoints, linear or logistic mixed models are used, depending on the blazon of outcome (numerical or binary, respectively). Furthermore, the same stock-still furnishings as for the primary issue are included.
Discussion
Other studies have mainly focused on surrogate outcomes every bit primary endpoint. These endpoints, such every bit reduction of drugs, MAI or drug costs, fail at showing clinical outcomes [10,26,27,28,, 12, 15, 26–29]. In the present study, we are focusing both on hard endpoints (i.e. patient relevant outcomes), and surrogate outcomes. The principal endpoint, withal, is a combined set of difficult endpoints with a clear clinical result. For this reason, the duration of this study is one twelvemonth; other studies not using hard endpoints have shorter written report periods [vi, 8, 10, 29]. Furthermore, this study is a multicentre study including over 3000 patients making it a relatively large written report in comparing with other studies [6, eight, ten, 27, 28].
A major give-and-take point with other articles is the fact that a swell number of studies focus on reducing the corporeality of prescribed drugs whereas the focus should exist on optimising the prescribed drugs (rationalistic pharmacotherapy). This fact enlightens the paradoxically relation between polypharmacy and underprescribing as it might be confronting to add new medication to an already polymedicated patient whereas reducing medication might seem the most logical mode to perform [ix]. For some patients, optimising the medication will imply reducing the number of drugs, for other patients it will be the changing of some drugs or adding some drugs [xxx].
We strongly believe that past using a CCDSS, medication reviews are performed in a standardised mode which leads to comparable results between patients. In improver, using a CCDSS eliminates the fourth dimension factor to perform medication reviews equally the major issues related to medication, laboratory values, indications and/or established patient characteristics will be directly available. In this fashion, and in order to brand the medication review process complete, consultation inside healthcare professionals and/or the patient itself will be fourth dimension effective and the medication surveillance could be performed effectually the clock. Especially for polymedicated patients, like nursing home patients, this system provides a paw full of advantages to provide continuous surveillance, improving in this manner patient care.
Abbreviations
- CCDSS:
-
(Computerised clinical conclusion support system)
- CRR:
-
(Clinical rule reporter)
- ICC:
-
(Intra-course correlation coefficient)
- IGZ:
-
(Inspectie voor de gezondheidszorg/dutch wellness inspectorate)
- MAI:
-
(Medication appropriate index)
- METC:
-
(Medisch Ethische toetsingscommissie/medical ethical committee)
- SCREAM:
-
(Supporting clinical rules engine in the adjustment of medication)
- SCREEN:
-
(Supporting clinical rules in the evaluation of elderly patients with neuropsychiatric disorders)
References
-
Finkers F, Maring JG, Boersma F, Taxis K. A study of medication reviews to place drugrelated problems of polypharmacy patients in the Dutch nursing dwelling setting. J Clin Pharm Ther. 2007;32:469–76.
-
Nguyen JK, Fouts MM, Kotabe SE, Lo E. Polypharmacy as a take chances cistron for adverse drug reactions in geriatric nursing home residents. Am J Geriatr Pharmacother. 2006;4:36–41.
-
Multidisciplinaire Richtlijn Polyfarmacie bij ouderen 2012. https://www.nhg.org/sites/default/files/content/nhg_org/uploads/polyfarmacie_bij_ouderen.pdf. Accessed Nov 2014.
-
https://www.sfk.nl/nieuws-publicaties/PW/2012/polyfarmacie-voor-i-op-de-ten-apotheekbezoekers. Accessed 30 Jul 2013.
-
Field TS, Gurwitz JH, Avorn J, McCormick D, Jain Due south, Eckler M, et al. Risk factors for adverse drug events among nursing home residents. Arch Intern Med. 2001;161:1629–34.
-
Crotty Thou, Halbert J, Rowett D, Giles L, Birks R, Williams H, et al. An outreach geriatric medication advisory service in residential anile intendance: a randomised controlled trial of case conferencing. Age Ageing. 2004;33:612–7.
-
Stuijt CCM, Franssen EJF, Egberts ACG, Hudson SA. Appropriateness of prescribing among elderly patients in a Dutch residential home: observational report of outcomes afterward a pharmacist-led medication review. Drugs Aging. 2008;25:947–54.
-
Furniss L, Burns A, Craig SK, Scobie S, Cooke J, Faragher B. Effects of a pharmacist'southward medication review in nursing homes. Randomised controlled trial. Br J Psychiatry. 2000;176:563–vii.
-
Kuijpers MAJ, van Marum RJ, Egberts ACG, Jansen PAF, The OLDY (Sometime people Drugs & dYsregulations) written report group. Relationship betwixt polypharmacy and underprescribing. Br J Clin Pharmacol. 2007;130:130–3.
-
Zermansky AG, Alldred DP, Petty DR, Raynor DK, Freemantle N, Eastaugh J, et al. Clinical medication review by a pharmacist of elderly people living in care homes—randomised controlled trial. Age Ageing. 2006;35:586–91.
-
Roberts MS, Stokes JA, King MA, Lynne TA, Purdie DM, Glasziou PP, et al. Outcomes of a randomized controlled trial of a clinical chemist's intervention in 52 nursing homes. Br J Clin Pharmacol. 2001;51:257–65.
-
Holland R, Desborough J, Goodyer L, Hall S, Wright D, Loke YK. Does chemist-led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta-assay. Br J Clin Pharmacol. 2007;65:303–xvi.
-
Holland R, Lenaghan E, Harvey I, Smith R, Shepstone L, Lipp A, et al. Does dwelling house based medication review keep older people out of hospital? The HOMER randomised controlled trial BMJ. 2005;330:293.
-
Gurwitz JH, Soumerai SB, Avorn J. Improving medication prescribing and utilization in the nursing home. J Am Geriatr Soc. 1990;38(v):542–52.
-
Patterson SM, Hughes C, Kerse North, Cardwell CR, Bradley MC. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev 2012;(v)CD008165.
-
Lepane KL, Hughes CM, Daiello LA, Cameron KA, Feinberg J. Effect of a pharmacist-led multicomponent intervention focusing on the medication monitoring stage to prevent potential adverse drug events in nursing homes. J Am Geriatr Soc. 2011;59:1238–45.
-
Allard J, Hebert R, Rioux G, Asselin J, Voyer L. Efficacy of a clinical medication review on the number of potentially inappropriate prescriptions prescribed for community-habitation elderly people. CMAJ. 2001;164:1291–6.
-
Richmond S, Morton Five, Cross B, Chi Kei Wong I, Russell I, Philips Z, et al. Effectiveness of shared pharmaceutical intendance for older patients: RESPECT trial findings. Br J Gen Pract. 2010;59:14–20.
-
Krska J, Cromarty JA, Arris F, Jamieson D, Hansford D, Duffus PR, et al. Pharmacist-led medication review in patients over 65: a randomized, controlled trial in master care. Age Ageing. 2001;thirty:205–11.
-
Schnipper JL, Kirwin JL, Cotugno MC, Wahlstrom SA, Dark-brown BA, Tarvin Due east, et al. Pharmacist counselling in preventing adverse drug events after hospitalization. Curvation Intern Med. 2006;166:565–71.
-
Pearson SA, Moxey A, Robertson J, Hains I, Williamson M, Reeve J, et al. Practise computerised clinical decision back up systems for prescribing change practice? A systematic review of the literature (1990–2007). BMC Health Serv Res. 2009;9:154.
-
Hemens BJ, Holbrook A, Tonkin M, Mackay JA, Weise-Kelly L, Navarro T, et al. for the CCDSS Systematic Review Squad. Computerized clinical decision support systems for drug prescribing and management: a conclusion-maker-researcher partnership systematic review. Implementation Scientific discipline. 2011;6:89.
-
Roshanov PS, Misra S, Gerstein HC, Garg AX, Sebaldt RJ, Mackay JA, et al. for the CCDSS Systematic Review Team. Computerized clinical determination support systems for chronic disease management: A decision maker-researcher partnership systematic review. Implementation Scientific discipline. 2011;6:92.
-
de Wit HA, Mestres Gonzalvo C, Hurkens KP, Mulder WJ, Janknegt R, Verhey FR, et al. Evolution of a computer arrangement to support medication reviews in nursing homes. Int J Clin Pharm. 2013;35:668–72.
-
Hurkens KPGM, MestresGonzalvo C, de Wit HAJM, van der Kuy PHM, Janknegt R, et al. A survey on medication reviews in older patients: substantial variation in daily practise. J Gerontol Geriat Res. 2013;2:133. doi:10.4172/2167-7182.1000133.
-
Kaur Southward, Mitchell 1000, Vitetta L, Roberts MS. Interventions that tin reduce inappropriate prescribing in the elderly: a systematic review. Drugs Aging. 2009;26:1013–28.
-
Male monarch MA, Roberts MS. Multidisciplinary example conference reviews: improving outcomes for nursing home residents, carers and health professionals. Pharm Globe Sci. 2001;23:41–v.
-
Garfinkel D, Zur-Gil S, Ben-Israel J. The state of war against polypharmacy: a new cost-effective geriatric-palliative approach for improving drug therapy in disabled elderly people. Isr Med Assoc J. 2007;ix:430–4.
-
Pope Yard, Wall N, Peters CM, O'Connor M, Saunders J, O'Sullivan C, Donnelly TM, Walsh T, Jackson S, Lyons D, Clinch D. Specialist medication review does not benefit short-term outcomes and net costs in continuing-care patients. Historic period Ageing. 2011;40:307–12.
-
Kwan D, Farrell B. Polypharmacy—optimizing medication use in elderly patients. Pharm Pract. 2012;29(ii):20–5.
Acknowledgements
Not applicable.
Funding
The complete SCREEN project, which includes the SCREAM written report, is supported by a grant from the ZonMw (the netherlands Organization for Wellness Research and Evolution). [Grant number: 113101001].
Availability of information and materials
Not applicative.
Authors' contributions
All authors have made substantial contributions to formulation and blueprint, acquisition of data, analysis and interpretation of data; They all take been involved in drafting the manuscript and revising information technology critically for important intellectual content; They all accept given final approving of the version to be published; and they all concord to exist accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and canonical the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicative.
Ethics approval and consent to participate
The study protocol has been canonical by the Medical Upstanding Committee (METC). The risks of participation are negligible and the burden can be considered minimal as the report will only create advices towards the md and he will exist the one deciding whether it is applicable or not. All the advices that the program creates are testify based and rely on upwardly-to-date guidelines or literature. Good clinical practice will exist maintained during the whole study.
Author data
Affiliations
Corresponding author
Rights and permissions
Open Access This commodity is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in whatever medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Artistic Commons license, and indicate if changes were made. The Artistic Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/naught/ane.0/) applies to the data fabricated available in this commodity, unless otherwise stated.
Reprints and Permissions
Almost this article
Cite this article
Mestres Gonzalvo, C., de Wit, H.A.J.M., van Oijen, B.P.C. et al. Supporting clinical rules engine in the aligning of medication (SCREAM): protocol of a multicentre, prospective, randomised report. BMC Geriatr 17, 35 (2017). https://doi.org/ten.1186/s12877-017-0426-three
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s12877-017-0426-iii
Keywords
- Polypharmacy
- Medication therapy direction
- Decision support systems management
- Anile
- Medication review
Source: https://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-017-0426-3
0 Response to "Charge to Review and Adjusting Medications in a Clinic"
Post a Comment